Coastal area is the place where seawater is commingled with river water. In additional, coast water is rich in suspended particles originated from soil and sedimentary materials. The complicated pattern in water density and water chemistry properties at aquatic coastal area can result in particle deposition. The deposited particles can lead to various environmental hazards including an increase in seabed elevation, water quality deterioration and reduction in processing facility throughput. Among many types of deposited particles, inorganic mineral scale particles can form when a local superstation is achieved with respect to inorganic solids. Other than throughput reduction, the formed mineral scale particles can also deposit onto equipment surface, resulting in equipment efficiency reduction and malfunction. Extensive studies have been carried out to evaluate the deposition thermodynamics of solids including scale solids, via experimental and computational approaches. However, there are still limited studies on solid particle deposition kinetics. Deposition kinetics can provide the critical information as of how fast solid particles can precipitate and deposit on the surrounding environmental surfaces.
With the understanding of the solid particle deposition kinetics, efforts will be made to prepare and test functional nanomaterials for solid especially mineral scale particle deposition control. These functional nanomaterials are embedded with chemical inhibitor which can delay scale deposition kinetics in aqueous phase. Compared with conventional materials, these functional nanomaterials are expected to have a higher solid deposition control efficiency and also enhanced transport properties in various environmental media.

Mineral scale solid deposition and its environmental impact.
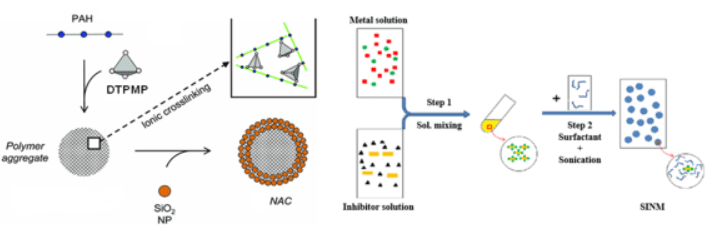
Synthesis of functional nanomaterials for solid particle deposition control.
Related publications:
- Zhang, P., Zhang, Z., Zhu, J., Kan, A.T., Tomson, M.B. (2019). Experimental evaluation of common sulfate mineral scale co-precipitation kinetics in oilfield operating conditions. Energy Fuels. 33, 7, 6177-6186.
- Zhang, P., Liu, Y., Kuok, S.C., Kan, A.T. & Tomson, M.B. (2019). Development of modelling approaches to describe mineral scale deposition kinetics in porous medium and pipe flow system. Journal of Petroleum Science and Engineering, 178, 594-601.
- Zhang, P., Huang, S., Zhang, N., Kan, A.T., Tomson, M.B. (2019). Automated analytical method to determine solution alkalinity of oilfield brine in the presence of weak organic acids. Ind. Eng. Chem. Res. 58, 4667−4673.
- Zhang, P., Zhang, N., Liu, Y., Ip, W.F., Kan, A.T. & Tomson, M.B. (2019). A novel attach-and-release mineral scale control strategy: Laboratory investigation of retention and release of scale inhibitor on pipe surface. Journal of Industrial and Engineering Chemistry, 70, 462-471.
- Zhang, P., Zhang, N., Liu, Y., Lu, Y-T., Kan, A.T. & Tomson, M.B. (2018). Application of a novel tube reactor for investigation of calcium carbonate mineral scale deposition kinetics. Chemical Engineering Research and Design, 137, 113-124.
- Zhang, Z., Zhang, P., Li, Z., Kan, A.T. & Tomson, M.B. (2018). Laboratory evaluation and mechanistic understanding of the impact of ferric species on oilfield scale inhibitor performance. Energy Fuels, 32, 8348-8357.
- Zhang, P., Shen, D., Ruan, G., Kan, A.T. & Tomson, M.B.* (2017). Phosphino-polycarboxylic acid modified scale inhibitor nanomaterial: Synthesis, characterization and migration. Journal of Industrial and Engineering Chemistry, 45, 366–374
- Zhang, P., Shen, D., Kan, A.T. & Tomson, M.B.* (2016). Mechanistic understanding of calcium-phosphonate solid dissolution and scale inhibitor return behavior in oilfield reservoir: formation of middle phase. Physical Chemistry Chemical Physics, 18, 21458-21468
